Navigating safety reporting compliance during the EU transition from CTD to CTR
As the European Union progresses from the Clinical Trials Directive (CTD) to the Clinical Trial Regulation (EU No 536/2014, CTR), it is imperative for stakeholders to grasp the evolving safety reporting requirements. From January 31, 2025, the CTR will exclusively govern all clinical trials, superseding the CTD as per Article 98. Clinical trials continuing under the CTD beyond this date must transition to the CTR framework to remain compliant. Failure to transition will result in non-compliance, potentially leading to corrective measures, penalties, and civil or criminal liability under Articles 77, 94, and 95 of the CTR.
The transition process: From CTD to CTR
Transitioning clinical trials from the CTD to the CTR involves an administrative process requiring a "transitioning application submission." Member States will conduct a minimal assessment to ensure compliance with key CTR principles, such as transparency and trial categorization. Sponsors are strongly encouraged to register their clinical trials under the Clinical Trials Information System (CTIS) as early as possible, considering the time required for the approval of transitioning applications.
Key Principles for Transition
Safety reporting requirements: During and after the Transition
The safety reporting obligations to national competent authorities (NCAs) and ethics committees (ECs) differ depending on whether the trial is governed by the CTD or the CTR during this transitional phase.
Reporting Suspected Unexpected Serious Adverse Reactions (SUSARs)
Development Safety Update Report (DSUR) submission
For CTD Trials: DSURs must be submitted directly to the Member State that authorized the trial.
Reporting to Ethics Committees (ECs)
Key features and timelines of CTR
Centralization
The CTR ensures uniform application across the EU by not requiring national implementation, unlike directives. This centralization simplifies the regulatory environment and fosters a more cohesive approach to clinical trials across member states.
Clinical Trials Information System (CTIS)
CTIS serves as a central hub for all clinical trial submissions, significantly reducing administrative burdens by eliminating the need for parallel processes and enhancing communication between sponsors, Member States, and the European Medicines Agency (EMA).
Key Milestones
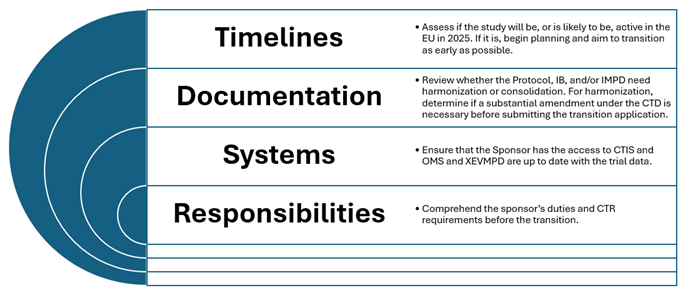
Detailed considerations for sponsors
Ensuring Timely Transition
Sponsors must prioritize transitioning their clinical trials to the CTR framework before the January 31, 2025 deadline. This involves:
Adapting to new safety reporting protocols
Understanding the nuances of safety reporting under both the CTD and CTR is essential for compliance:
Leveraging technology and expertise
Utilizing the CTIS for centralized submissions not only simplifies the process but also enhances communication and transparency. Sponsors should invest in training and technology to navigate this system efficiently.
Seeking assistance with safety reporting?
Navigating the complexities of safety reporting during this transition can be challenging. AWINSA offers expert guidance and support to ensure compliance with both CTD and CTR requirements. Our services include:
Contact AWINSA today to learn how we can help you manage your safety reporting obligations efficiently, ensuring your clinical trials remain compliant with evolving regulations.
Copyright © 2024 AWINSA Life Sciences Pvt. Ltd. All rights reserved.